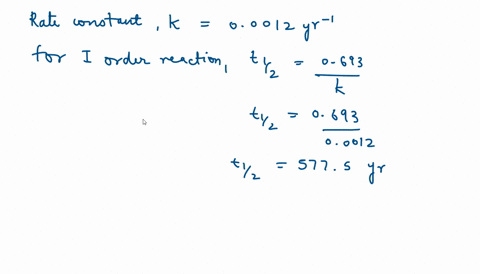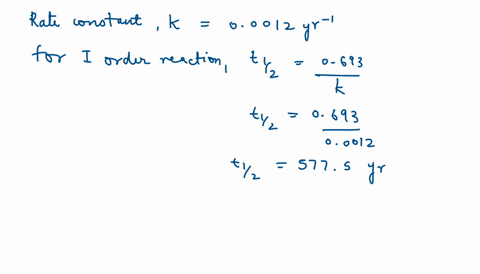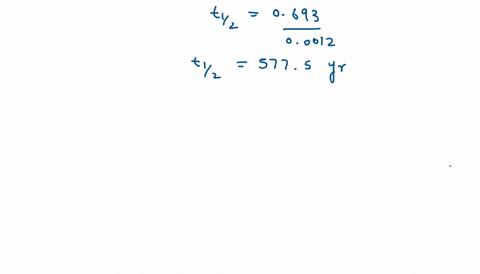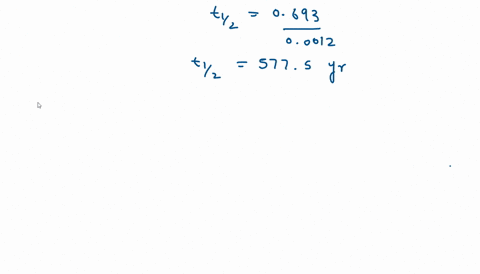Solve this: Q 5 In a first order reaction, the rate constant is 0 693 hr-1 The half-life - Chemistry - Chemical Kinetics - 12322113 | Meritnation.com

A first order reaction is half complete in 45 min. Caluclate the rate constant for the reaction - Find 6 Answers & Solutions | LearnPick Resources

SOLVED:The following data have been obtained for the decomposition of N2 O5(g) at 67^∘ C according to the reaction 2 N2 O5(g) →4 NO2(g)+O2(g) Identify the order of the reaction with respect

Calculate the half life of the first order reaction from their rate constant given as a) 200s^(-) b) 2min^(-1) c) 4 "year"^(-1).

The half - life period for a first order reaction is 693 seconds. The rate constant for this reaction would be:

Half life of a first order reaction is 2.1xx10^(12)s. Calculate the rate constant of the reactio... - YouTube

The rate constant of a reaction is 0.69 xx 10^(-1) and the initial concentration is 0.2 "mol l"^(-1). The half-life period is

OneClass: Half-life equation for first-order reactions: t1/2= .693/k where t1/2 is the half-life in s...
✓ Solved: The rate constant for a certain radioactive nuclide is 1.0 × 10^-3 h^-1 .What is the half-life...

The reaction `A(g)toB(g)+2C(g)` is a first order reaction with rate constant `2.772xx10^(-3)sec^(-1) - YouTube

The rate constant of a reaction is `0.0693 min^(-1)`. Starting with `10 mol`, the rate of the re... - YouTube

A first order reaction is found to have a rate constant k= 5.5 xx 10^(-14)s^(-1). Find half-life of the reaction.

Chemical Kinetics Class 12 Notes Chemistry Chapter 4 - Learn CBSE | PDF | Reaction Rate | Reaction Rate Constant

Define half life of a reaction Derive the relationship between half life and rate constant for a first Order - Chemistry - - 16068871 | Meritnation.com