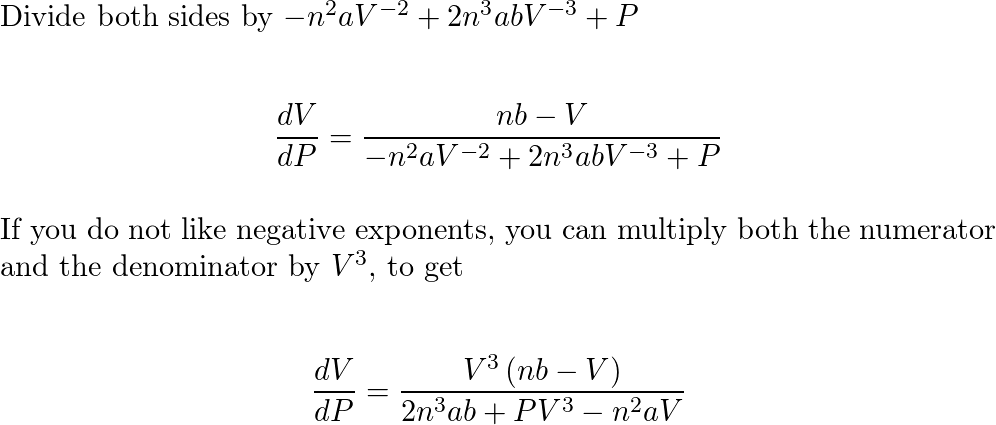Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =

A good coilover kit for an excellent price. Fixed damping matched to the springs. Exclusive to FM! – flyinmiata

The equation of state of n moles of a non - ideal gas can be approximated by the equation (P + an^2V^2)(V - nb) = nRT where a and b are constants

In the Van der Waals equation (P + (n^(2)a)/(V^(2))) (V-nb) = nRT, why is the term n^(2)a//V^(2) positive in sign.
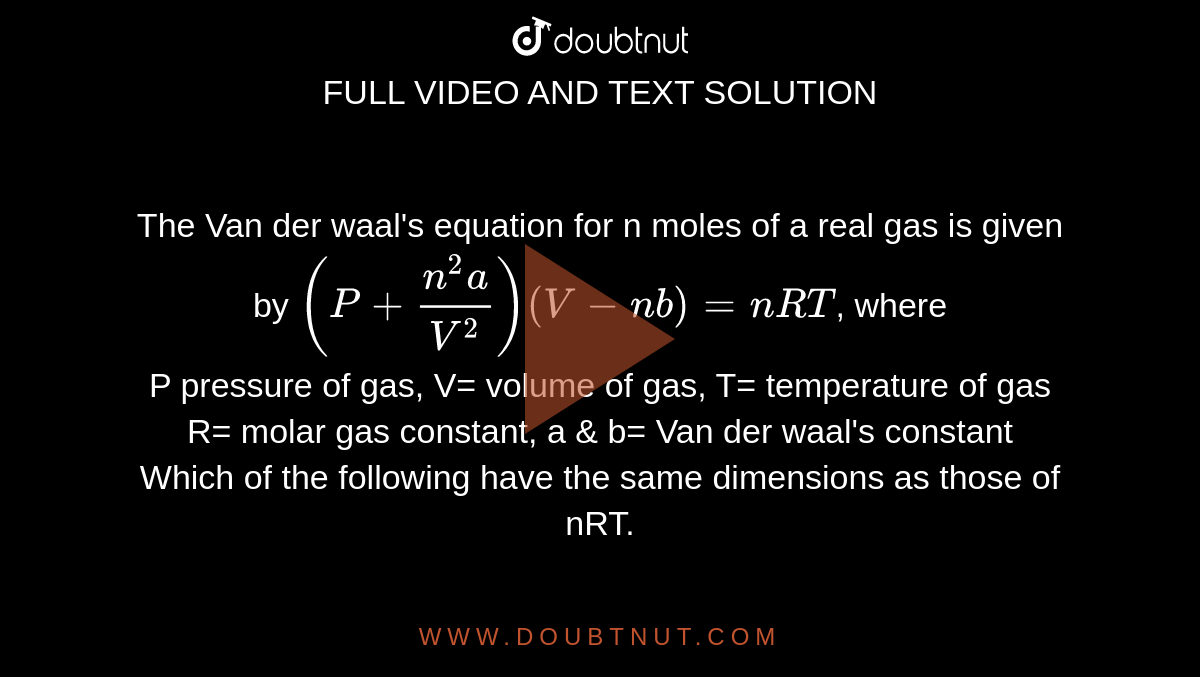
The Van der waal's equation for n moles of a real gas is given by (P+(n^(2)a)/V^(2)) (V-nb)=nRT, where P pressure of gas, V= volume of gas, T= temperature of gas R= molar

SOLVED:(a) The van van der Waals equation for n moles of a gas is (P + (n^2 a)/(V^2))(V - nb) = nRT where P is the pressure, V is the volume, and
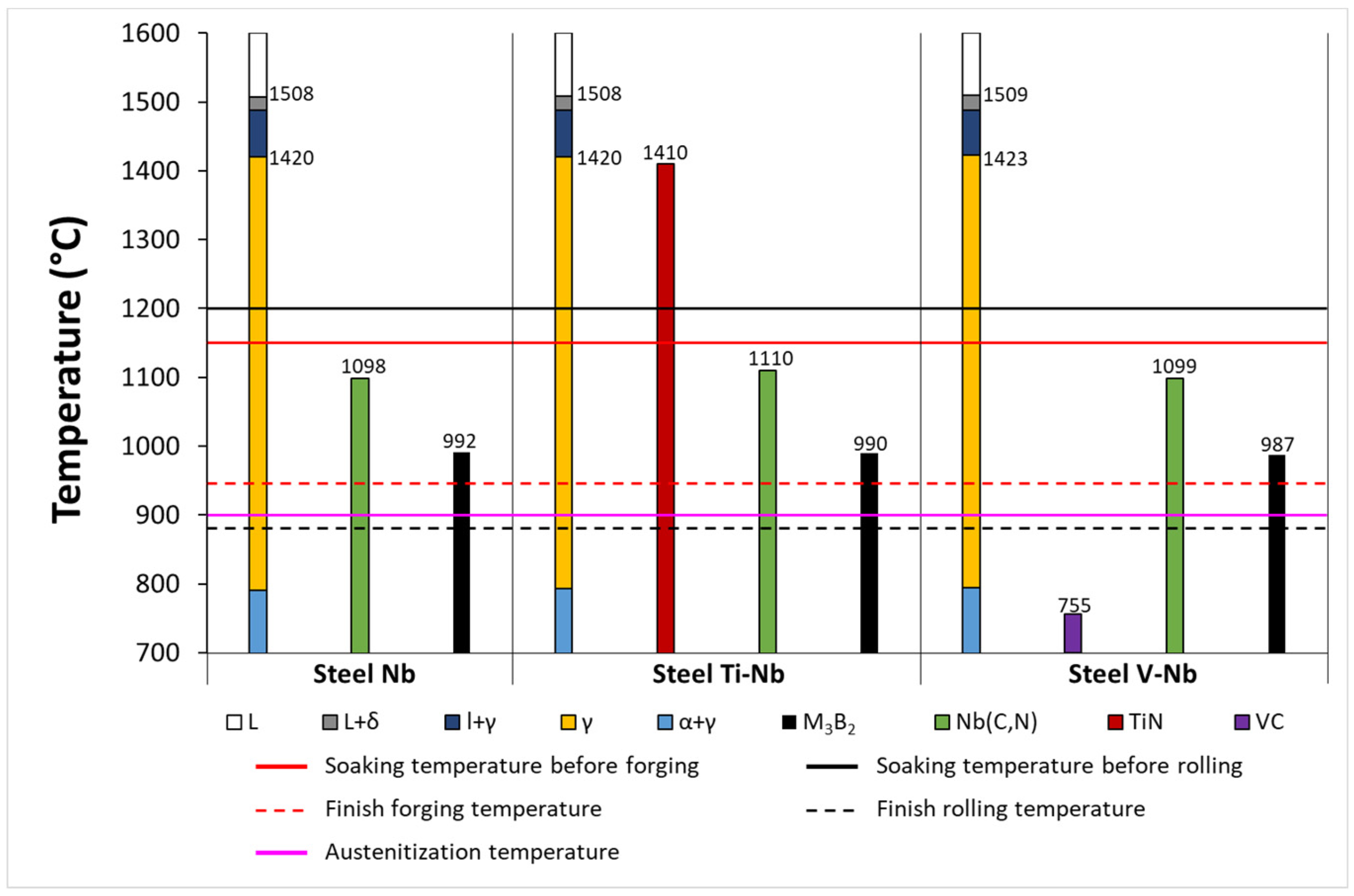
Metals | Free Full-Text | Grain Size Evolution and Mechanical Properties of Nb, V–Nb, and Ti–Nb Boron Type S1100QL Steels

The equation of state of n moles of a non - ideal gas can be approximated by the equation (P + an^2V^2)(V - nb) = nRT where a and b are constants





![Solved P= aN2 V2' 2-2. [20 points] Using the van der Waals | Chegg.com Solved P= aN2 V2' 2-2. [20 points] Using the van der Waals | Chegg.com](https://media.cheggcdn.com/media/eeb/eebc5207-da0f-4603-8b84-5abbdf4904c6/phpBa9k8H.png)